
Honey has since ancient times been used in the traditional treatment of wounds and burns. During the last years the use of honey for these indications has reemerged, mainly due to clinical observations of antimicrobial effect and accelerated wound healing [1,2]. The mechanisms of action ascribed to the effects of honey are largely unknown, but they are speculated to be related to osmotic activity, pH, hydrogen peroxide release, specific plant derived factors, and miscellaneous factors such as maintaining a moist wound environment [3, 4].
In order to validate the use of honey in medical treatment, understanding the mechanism of action seems crucial since the compositions of this natural product varies. In recent years several groups have examined honey in order to elucidate its antimicrobial properties. It has been suggested that the wound healing effect of honey may in part be related to the release of inflammatory cytokines from surrounding tissue cells, mainly monocytes and macrophages. Immunomodulatory effects were demonstrated in vitro by cytokine release from the monocytic cell lines and human peripheral monocytes after incubation with honey [5]. The findings show that natural honeys can induce interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) release.
Unadulterated alpine honey called Anushak Bio honey is produced from the nectar of hundred and one alpine plants, including Sage, Marjoram, Cephalaria, Thyme, Mint, Shepherd’s-club, St John’s wort, etc. It has not been treated by thermal or any other method and thus ANUSHAK BIO honey stand out by its excellent nutritional properties. In addition Anushak Bio honey enriched with Bess milk (Royal Jelly) at 2:100 proportion to honey is an irreplaceable source of natural compounds, including amino acids, vitamins, proteins and microelements for the human well being. Dry matter chemical analysis using HPLC, NMR and atomic absorption spectroscopy reveals that per 100 g the Anushak Bio honey contains 50.3 g fructose, 31.2 g glucose, 0.7 g sucrose 1.6 g saturated fat, 3.5 g total protein, 82.3 g total carbohydrate, < 2 mg cholesterol, 2.69 mg sodium, 9.98 mg calcium, 3.84 mg iron and no heavy metals, including As, Cd, Hg and Pb as well as pesticides has been detected.
Thisstudy was undertaken to determine the effects of Anushak Bio Honey (ABH) and enriched with Royal Jelly BIO Honey (RJ-ABH) on the human monocytes activation and release of IFN-γ, TNF-α, IL-1β and IL-10 and human monocyte-derived dendritic cells activation in vitro. Effects on these important pro-and anti-inflammatory responses may serve to explain the expected immunomodulatory and wound-healing properties of Anushak honey.

Honey solutions. Anushak Bio Honey (ABH) and enriched with Royal Jelly BIO Honey (RJ-ABH) were diluted to 10% w/v with pyrogen-free water and stored at 2–5ºC. The artificial honey (AH) was prepared by dissolving 50.3 g fructose, 31.2 g glucose, 0.7 g sucrose 1.6 g in 100 ml pyrogen-free water and stored at 2–5 ºC. The 10% stock solutions were used only on the day of preparation.
Endotoxin determination by the limulus amebocyte lysate assay. The content of endotoxin in the honey products was tested in a kinetic-turbidometric LAL assay (Test for bacterial endotoxins). Solutions (0.1%) of the honeys were prepared in pyrogen-free water and assayed by the LAL test as described in the European Pharmacopoeia [6]. In brief, the method was based on the quantitative relationship between the endotoxin concentration and the time (onset time) needed for the reaction mixture to reach a predetermined optical density. The LAL reagent Pyrotell-T (Associates of Cape Cod, USA) was used and an E. coli O55:B5 endotoxin 222 pg/EU (Cambrex Bioscience, USA) used as endotoxin standard. A standard curve in the range 12.5–10,000 pg/ ml was used.
Peripheral blood samples. Heparinised peripheral blood was obtained from 6 normal donors (3 male and 3 female). No significant differences existed between healthy donors with respect to mean levels of ESR and WBC counts.
Monocytes isolation and activation. Peripheral blood mononuclear cells (PBMC) were separated from heparinized whole blood by ficoll-hypaque (histopaque) (Sigma Chemical Co., St. Louis, MO) density gradient centrifugation. Peripheral blood monocytes isolated by cell adherence of PBMC to 25 cm2 plastic flasks during 45 min incubation at 37ºC in atmosphere, containing 6% CO2 [7]. Monocytes (95% CD14 positive cells) were then washed three times with endotoxin-free PBS and cultured at 5 ×105 cells/ml density in RPMI-1640 containing 10% FCS, 2mM L-glutamine, 1mM sodium pyruvate, 100 U/ml penicillin and 100 μg/ml streptomycin. Monocytes treated either with 0.2–1.0 % diluted ABH, RJ-ABH or 100 ng/ml LPS (positive control) or 0.2–1.0 % diluted AH (negative control) and cells were incubated during 24, 48 and 72 h at 37 ºC with 5% CO2. After incubation cells were pelleted by centrifugation and cell-free supernatants were collected and stored at -80 ºC. IFN-γ, TNF-α, IL-1β and IL-10 concentration in culture supernatants was determined by conventional ELISA using Ready-SET-Go test kits (eBioscience) with detection limit 2 pg/ml, accords the manufacturer recommendations.

Generation of monocyte-derived dendritic cells (DCs). For generation of immature DCs (iDCs), plastic adherence monocytes were cultured for 6 days with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) as it originally described by Morse et al. [8]. Mature DCs (mDCs) in vitro generated by monocytes incubation with GMCSF (10 ng/ml), IL-4 (10 ng/ml) and pro-inflammatory TNF-α (10 ng/ml) and cultured for 6 days [8]. One day 6, cells washed and fresh medium added or stimulated with 100 ng/ml LPS or 1.0 % diluted ABH and RJ-ABH or AH for additional 24 h. Cell free supernatants decanted and stored at -80°C and IL-10 concentration in samples determined by ELISA.
Flow cytometry. Monocyte-derived iDCs and mDCs were washed and surface stained either by 0.3 μg biothin-conjugated anti-CD86 (eBioscience) followed by PE-conjugated Streptavidin or PE-conjugated anti-CD14 followed by Streptavidin-FITC (eBioscience) or matched isotype controls. Cells were fixed in 1% paraformaldehyde and subjected to FACS analysis on a Becton Dickinson FACSCalibur flow cytometer with CellQuest™ software (Becton Dickinson, San Jose, CA, USA).

The endotoxin contents of the honeys are displayed in Table 1. All natural honeys contained significant amounts of endotoxin ranging from 284 ± 11 to 288 ± 21 ng endotoxin/g honey. There was no any significant differences were found in endotoxin contents between ABH and RJ-ABH. The artificial honey however did not contain a detectable amount of endotoxin in the dilution tested (0.1% w/v) which means that artificial honey contains less than 5 ng endotoxin/g honey.

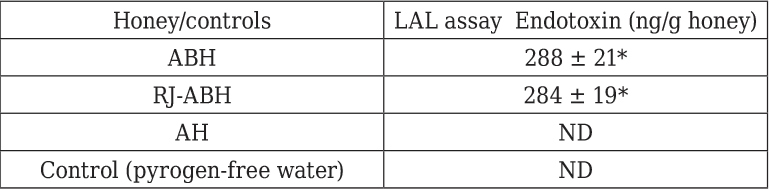
Results are displayed as mean ± SEM (n = 6). * Significant different from control (p < 0.05). ND, not detectable. The assay was conducted on 0.1% solutions of the honeys diluted with pyrogen-free water.

We studied if the ABH and RJ-ABH caused stimulation of IL1β production by normal human monocytes in vitro. Monocytes were incubated during 18 h in the presence of 0.25, 0.5 and 1.0 % dilution of either ABH and RJ-ABH and positive control LPS and negative control AH and IL-1β concentration in culture.
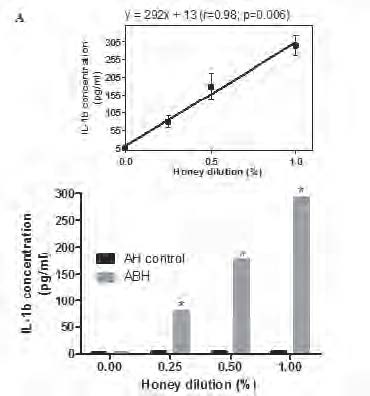
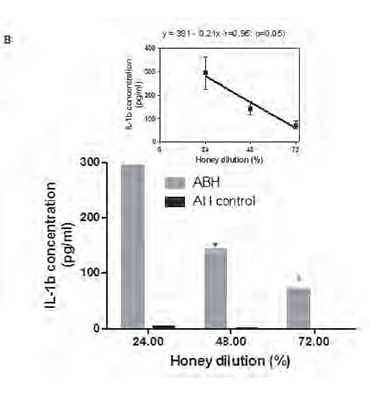

Monocytes were incubated during 18 h in the presence of 0.25, 0.5 and 1.0 % dilution of either ABH or negative control AH (A) or with 1.0 % dilution of either ABH or negative control AH during 24, 48 and 72 h (B), as described in section Material and methods. IL-1β concentration in cell free supernatants determined by ELISA. Linear regression curves (upper boxed graphs) and equations (where y - is the IL-1β concentration and x - is the Id 1F7 concentration or time) were generated from calculated mean values ± SD data for honey concentration using Graph Pad Prism v4.01 software. All data represent means ± SD (error bars) and are significantly different comparing AH control (* Pt < 0, 05).
supernatants determined by conventional ELISA. We found that ABH at all tested concentrations induced significant (P=0.01) up-regulation of IL-1β production in all 6 individuals tested. This effect was dose-depended as liner regression assay (Fig.1A) revealed the equation y=292x + 13 (r=0.98; p=0.006), where y - is the IL-1β concentration and x - is the ABH dilution. ABH time-dependent action on IL-1β production by monocytes was studied using 1.0 % dilution of ABH which was selected because of its highest effect on IL-1β secretion. ABH-treated cells were incubated during 24, 48 and 72 h and IL-1β concentration in culture supernatants determined by ELISA.
Time course studies (Fig. 1B), have shown that maximal IL-1β concentration was detected at 24 h (294.2±68.3 pg/ml), with gradual decrease (p=0.034) at 48 h (13.1±27.9 pg/ml) and 72 h (72.1±17.5 pg/ml) as was revealed by non-parametric ANOVA for repeated measures.
Similar dose- and time-dependent results we obtained studying the stimulatory effect of RJ-ABH on IL-1β production by human monocytes, however we failed to show any significant differences comparing RJ-ABH with ABH. Table 2 summarizes the results we obtained on effects of different honey preparation as well as negative and positive controls on IL-1β production by human monocytes. From the presented in the Table 2
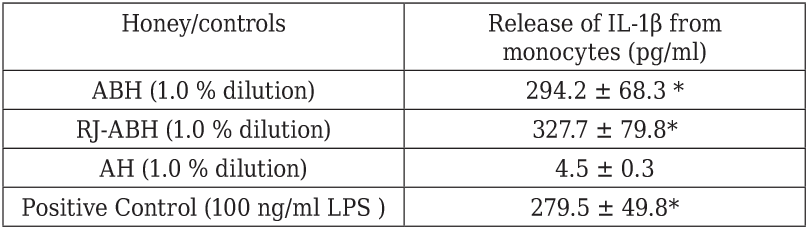
Peripheral blood monocytes stimulated either with 100 ng/ml LPS or 1.0 % dilution of ABH, RJ-ABH or AH for 24 h. Cell free supernatants decanted and IL-1β concentration in samples determined by ELISA. Results are displayed as mean ± SEM (n = 6). * Significant different from control (p < 0.05).
data follows that the stimulatory effect of both ABH and RJ-ABH on the release of IL-1β from monocytes can not be distinguish from the effect of LPS, taken as the positive control

Next, we studied if the ABH and RJ-ABH caused stimulation of TNF-α production by normal human monocytes in vitro. Monocytes were incubated during 18 h in the presence of 0.25, 0.5 and 1.0 % dilution of either ABH and negative control AH and TNF-α concentration in culture supernatants determined by conventional ELISA. We found that ABH at all tested concentrations induced significant (P=0.01) up-regulation of TNF-α production in all 6 individuals tested.
This effect was dose-depended as liner regression assay (Fig.2) revealed the equation y=576x + 18 (r=0.99; p=0.003), where y - is the TNF-α concentration and x - is the ABH dilution.
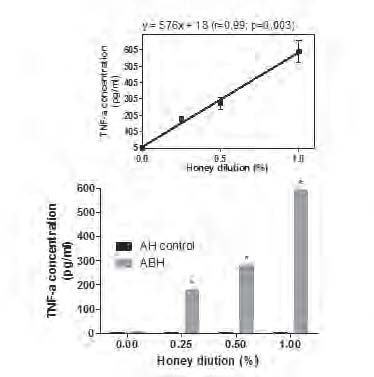
Monocytes were incubated during 18 h in the presence of 0.25, 0.5 and 1.0 % dilution of either ABH or negative control AH (as described in section Material and methods. TNF-α concentration in cell free supernatants determined by ELISA. Linear regression curves (upper boxed graphs) and equations (where y - is the IL-1β concentration and x - is the honey concentration) were generated from calculated mean values ± SD data for TNF-α concentration using Graph Pad Prism v4.01 software. All data represent means ± SD (error bars) and are significantly different comparing AH control (* Pt < 0, 05).
Next we studied if the ABH and RJ-ABH caused stimulation of IFN-γ, TNF-α and IL-10 production production by normal human monocytes in vitro. Monocytes were incubated during 18 h in the presence of 1.0 % dilution of either ABH and RJ-ABH and positive control LPS and negative control AH and cytokine concentration in culture supernatants determined by conventional ELISA. Cytokine production by monocytes was studied using 1.0 % dilution of honey which was selected because of its highest effect either on IL-1β and TNF-α secretion (Figures 1 and 2).

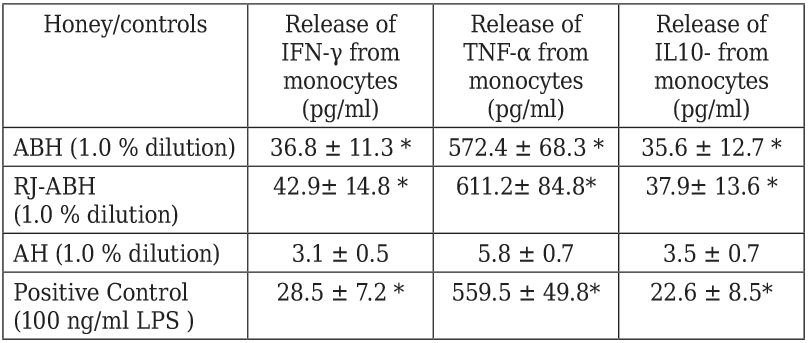
Peripheral blood monocytes stimulated either with 100 ng/ml LPS or 1.0 % dilution of ABH, RJ-ABH or AH for 24 h. Cell free supernatants decanted and cytokine concentration in samples determined by ELISA. Results are displayed as mean ± SEM (n = 6). * Significant different from control (p < 0.05).
Table 3 summarizes the results we obtained on effects of different honey preparation as well as negative and positive controls on IFN-γ, TNF-α and IL-10 production by human monocytes. From the presented in the Table 3 data follows that the stimulatory effect of both ABH and RJ-ABH on the release of IFN-γ, TNF-α and IL-10 from monocytes can not be distinguish from the effect of LPS, taken as the positive control. It should be noted that we failed to show any significant differences comparing stimulatory effects of both RJ-ABH with ABH on the release of IFN-γ, TNF-α and IL-10 from monocytes.

We evaluated the expression of CD86 and CD14 in unstimulated (AH-control-treated), LPS- and honey-treated DCs, generated from normal donors. As it expected, the CD86 surface expression is increased and the CD14 surface expression is decreased in mDCs, comparing with iDCs. Both LPS and honey stimulation of iDCs caused statistically significant (Pp < 0.02) increasing of CD86 surface expression compared with AH-treated cells. However, LPS and ABH (Pp < 0.003 and Pp < 0.01, respectively) but not RJ-ABH decreased mDCs CD86 surface expression (Figure 3).
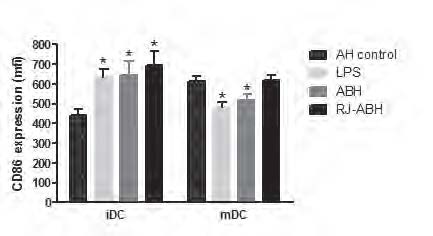
Monocyte-derived iDCs and mDCs, stimulated with 100 ng/ml LPS or 1.0 % dilution of ABH, RJ-ABH or AH for 24 h and surface stained by CD86 or matched isotype controls and subjected to FACS analysis and relative fluorescence intensities of CD86-PE expressed as a mean channel number (MCN). All data represent means ± SD (error bars) and are significantly different comparing with AH-treated control (* Pp< 0, 05).



We investigated two different types of honey. To elucidate the in vitro immunomodulatory effects of the various honeys, we employed two different cell assays based on monocytes. In the first, the monocyte release of IFN-γ, TNF-α, IL-1β and IL-10 in response to stimulation, which mimics the potential activation of monocytes and macrophages within the intact immune system was studied. As previously shown, the oxidant production and cytokine release from the monocytic cell lines and human peripheral monocytes was also affected by honey stimulation [5] and after incubation with natural honeys they induce IL-6, IL1β and TNF-α and activation of normal human monocytes. We investigated this separately using monocyte-derived dendritic cells activation in vitro cell assay [9]. In this assay, monocytes are differentiated along the immature or mature DCs. Since honey is a natural product it is very likely to contain remnants of bacteria. Because endotoxin from gram negative bacteria is a well-known potent immunomodulatory substance, we examined the content of endotoxin in the various honeys.
The data presented in this study indicate that the natural honey samples that were tested have a stimulatory effect with regard to the production of IFN-γ, TNF-α, IL-1β and IL-10 by human monocytes. The stimulatory effects of honey are unlikely to be an artefact of the human primary monocytes isolated from peripheral blood, since the responsiveness appears to be a property endogenous and the appropriate artificial honey negative control included the study reveals no stimulatory activity.
We investigated the possibility that honey-stimulated cytokine induction may be initiated by the presence of microbes or their components present as contaminants in honey. The low water activity and acidic nature of honey make it a generally unsuitable medium for bacterial growth [10]; nevertheless, a number of reports have described bacterial and fungal contamination of honey [11, 12]. The presence of bacteria raises the possibility that honey samples may be contaminated with bacterial components including LPS, which may be responsible for the cytokine inducing activity of the honey.
We assessed LPS concentrations in our honey samples and detected high levels of LPS (288 ng endotoxin/g honey). As LPS induces inflammatory cytokine production in monocytic cells, the stimulatory activity of the honey may be due to presence of endotoxins. Indeed, our data indicate a role for LPS in stimulating these inflammatory responses, but activity may be associated with bacterial or fungal components in origin present in honey, as these microbes are commonly isolated from manuka honey [11]. Whether the active components are of microbial origin or from some other source requires further investigation, and it is important to characterize the active molecules further. Our results would suggest that the regulatory effects of honey are related to components other than the sugars present, although the identity of the actual component(s) that mediate these effects are as yet unknown. Further studies, on the isolation and characterization of these agents, are required to further elucidate the mechanisms by which they exert their actions.

The inflammatory phase has an essential role in clearing the wound site of infection and debris and in initiation of the later stages of the wound-healing process [13]. In the present study, 2 different honeys were assessed for their ability to stimulate inflammatory cytokines. As illustrated in Table 1-3 all honey tested resulted in similar levels of cytokine induction. Although the wound healing activities of honey are well documented [14], little evidence exists regarding the scientific basis of the observed benefits associated with honey treatment. In particular, the component(s) responsible for the wound-healing activity and the mechanisms of action are yet to be identified. Whether honey affects other cell types, particularly endothelial cells and fibroblasts, involved in wound healing also needs to be clarified.
In summary, we report for the first time that the Aushak Bio Honey and enriched with Royal Jelly BIO Honey stimulates innate immune cells and particularly induce human monocytes activation and stimulate cytokine production in vitro. ArmApis BIO Honey stimulates the production of inflammatory cytokines including TNF-α, IL-1β as well as induces anti-inflammatory cytokine IL-10 and pleotropic immunoregulatory cytokine IFN-γ production by human monocytes.
Aushak Bio Honey and enriched with Royal Jelly BIO Honey induce up regulation of dendritic cells activation marker CD86 expression on monocyte-derived immature dendritic cells. These findings reveal mechanisms of Aushak Bio Honey andenriched with Royal Jelly BIO Honey stimulation of cytokine induction and could potentially lead to thedevelopment of novel therapeutics to improve wound healing for patients with acute and chronic wounds.
1. French VM, Cooper RA, Molan PC. The antibacterial activity of honey against coagulase-negative staphylococci. J Antimicrob Chemother 2005;56:228–31.
2. Molan PC. Potential of honey in the treatment of wounds and burns.Am J Clin Dermatol 2001; 2:13–9.
3. Lusby PE, Coombes A, Wilkinson JM. Honey: a potent agent for wound healing?. J Wound Ostomy Continence Nurs 2002; 29:295–300.
4. Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds 2006; 5:40–54.
5. Tonks AJ, Cooper RA, Jones KP, Blair S, Parton J, Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003;21:242–8
6. Council of Europe. Bacterial endotoxins. Ph Eur; 2005: p 1619, [Chapter 2.6.14.].
7. Procheray F, Viaud S, RimaniolL AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005; 142: 481-9.
8. Morse MA, Zhou LJ, Tedder TF, Lyerly HK, Smith C. Generation of dendritic cells in vitro from peripheral blood mononuclear cells with granulocyte-macrophage-colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha for use in cancer immunotherapy. Ann Surg. 1997l; 226(1): 6-16.
9. Timm M, Hansen EW, Moesby L, Christensen JD. Utilization of the human cell line HL-60 for chemiluminescence based detection of microorganisms and related substances. Eur J Pharm Sci 2006;27:252–8
10.Henriques, A., Jackson, S., Cooper, R., Burton, N. (2006) Free radical production and quenching in honeys with wound healing potential. J.Antimicrob. Chemother. 58, 773–777.
11.Snowdon, J. A., Cliver, D. O. Microorganisms in honey. Int. J. Food Microbiol. 1996, 31, 1–26.
12.Nakano, H., Sakaguchi, G. An unusually heavy contamination of honey products by Clostridium botulinum type F and Bacillus alvei. FEMS Microbiol. Lett. 1991 63, 171–177.
13.Martin, P., Leibovich, S. J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607.
14. Cooper, R. A., Molan, P. C., Harding, K. GThe sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. . 2002, 93, 857–863.
Dr. Tigran K. Davtyan, PhD, ScD
Tel: +374 10 23-72-61
Fax: +374 10 28-07-33
Email: tigdav@excite.co